Teonanacatl
Halluncinogenic
Mushrooms
of North america

Extracts from the
Second International Conference
on Hallucinogenic Mushrooms,
help October 27-30, 1977
near Port Townsend, Washington
Edited by
Jonathan Ott and Jeremy Bigwood
Madrona Publishers, Seattle
1778
Part B
It was at the end of 1956 when a small report in a newspaper awakened my vivid interest. I read that American scientists had discovered that some Indian tribes in the remote mountains of southern Mexico were using certain kinds of mushrooms in their religious ceremonies and in their medical practices, still based on magical concepts. The witch doctors, or shamans, would eat these mushrooms in order to enter a trance, in which state they were capable of soothsaying and giving advice to ill people who came to consult them. The ingestion of these mushrooms evidently produced hallucinations, which would account for such a magical use.
Unfortunately, in this newspaper article there was no name or address of the discoverer of this mushroom cult, not even an indication of where the original reports had been published. I was very eager to get more detailed information on these hallucinogenic mushrooms, because I had been interested in hallucinogenic agents and the phenomenon of hallucinosis since 1943 when I had discovered LSD, an hallucinogenic agent of extraordinary potency.
LSD, which is the laboratory code name for lysergic acid diethylamide (Zysergraure-diathylamid) is also related to a mushroom, to the fungus Claviceps purpurea (Fries) Tulasne, which is parasitic on rye and other Gramineae. The rye grain attacked by the fungus develops into ergot, into dark purple-brown pegs projecting from the ripening ears. Biologically speaking, ergot, which consists of compactly interwoven hyphae, is a sclerotium (in which stage the fungus passes the winter). Ergot is a rich source of pharmaceutically valuable alkaloids (3). The nucleus of most ergot alkaloids is a tetracyclic indolic compound named lysergic acid. In the thirties and early forties, I was occupied, in the pharmaceutical-chemical research laboratories of Sandoz Ltd. in Basel, Switzerland, with the preparation of semi-synthetic derivatives of lysergic acid. Among many other derivatives, I also prepared lysergic acid diethylamide in 1938, with hopes of obtaining an analeptic, that is, a compound with circulatory-stimulant properties. But in preliminary tests this compound was not found to be of pharmacological interest. In order to promote a further, more detailed pharmacological investigation of lysergic acid diethylamide, I repeated the synthesis of this compound in 1943. An accidental observation in the course of this work led me to carry out a planned self-experiment with LSD, which resulted in the discovery of its extraordinary hallucinogenic activity. The specific hallucinogenic potency of LSD led this compound to become an important psychopharmacological tool, which soon found widespread application in biology, in brain research, in experimental psychiatry for the production of model psychosis, and in psychoanalysis and psychotherapy as a valuable pharmacological aid.
Our work with LSD was what brought the sacred mushrooms of Mexico to the Sandoz laboratory for chemical analysis, as will be described later. Coincidentally, it eventually turned out that a close chemical relationship exists between LSD and the active principles of the hallucinogenic mushrooms.
In February 1957 a letter arrived at Sandoz in Basel, via the firm’s Paris branch, from Professor Roger Heim, director of the Laboratoire de Cryptogamie of the Museum National d’Histoire Naturelie in Paris, asking if we would be interested in carrying out a chemical investigation of the Mexican sacred mushrooms. Professor Heim, a world-famous mycologist, had been invited by the American investigators R. Gordon Wasson and his wife Valentina Pavlovna to participate in 1956 in an expedition to southern Mexico, to the regions where the Wassons had, in preceding years, explored the ceremonial use of certain mushrooms by the native population. Heim had succeeded in identifying and classifying the most important mushrooms used for magical purposes by the Indians, and had later been able to cultivate some of these mushrooms in his laboratory in Paris. He wrote in his letter that two laboratories in the United States, Smith, Kline and French, and Merck, were also in possession of corresponding mushroom material and were subjecting it to chemical analysis, but so far without positive results. Furthermore, he informed us that chemical investigations of his mushrooms by one of his colleagues in the chemical department of the Museum National d’Histoire Naturelle had also been unsuccessful. Believing that in the laboratory where LSD had been discovered the knowledge of how to handle hallucinogenic substances would be available, he had decided to offer us his mushroom material for chemical analysis. I accepted this offer with enthusiasm.
Through the mediation of Professor Heim, I came into personal contact with R. Gordon Wasson. In the beautiful two- volume edition Mushrooms, Russia and History, which came out in 1957 (10), the Wassons gave a comprehensive report of their ethnomycological studies over 30 years, which culminated in the study of the ancient Mexican mushroom cult.
The ceremonial use and worship of mushrooms by the Indians of Central America must be very old. In Guatemala so-called ‘mushroom stones’ have been found. These are stones carved in the form of a pileate mushroom, on the stem of which the head, or entire figure, of a god, or demon, is depicted. The oldest specimens found are over 2000 years old. It is probable that the mushroom cult dates back to at least 2000 years before the conquest of Mexico by Cortes. The Spanish chroniclers who followed the conquistadors mentioned teonandcatl in their writings, an Aztec word which could be translated as ‘sacred mushroom,’ or ‘God’s flesh.’ From these sixteenth century reports, it can be seen that teonandcatl was not only ingested at social and festival occasions but also by priest-doctors and soothsayers. The mushroom god—which the Christian missionaries called a devil—endowed them with, besides other things, the ability to identify the causes of diseases and to indicate the ways in which they could be treated.
For some centuries these intriguing reports in the old chronicles were given surprisingly little attention, probably because they were regarded as extravagances of a superstitious age. However, between 1936 and 1938, European and American
investigators, Weitlaner, Reko, Johnson, and Schultes, ascertained that mushrooms were still being eaten for magical purposes by the natives in certain districts of southern Mexico. But as already mentioned, systematic studies of the mushroom cult were only begun some years later, by the Wassons. Between 1953 and 1962 they made several expeditions to the remote mountainous districts of southern Mexico to study the current use of the magic mushrooms. In the summer of 1955, R. Gordon Wasson was, for the first time, able to take active part in a secret nocturnal mushroom ceremony in Huautla de Jime’nez, Oaxaca, and probably was the first white man to ingest the holy mushrooms. This experience, which impressed him profoundly, has been described by him in detail in his monograph. As already mentioned, the Wassons, not being experts in the botanical aspects of mycology, asked Professor Heim to collaborate with them in order to identify the sacred mushrooms botanically.
Heim showed that these mushrooms were mostly new species of the family Strop hariaceae, the greater part belonging to the genus Psilocybe, as well as one species of the genus Stropharia and one species of the genus Conocybe. Artificial cultivation in the laboratory provided a very good yield, especially of one of these sacred mushrooms, namely Psilocybe mexicana Heim. (See color illustration 17.)
In the first half of 1957, Heim sent us for chemical analysis, in several portions, some one hundred grams of dried mushroom material referable to this species. As we had no indication as to what class of chemical compounds the active principles represented, we had to rely in our isolation experiments on activity tests. The extract fractions were first tested on animals. Studies were made of pupillary reaction and of piloerection in mice, and of general behavior in dogs. But the results were not clear-cut and led to disagreements in the evaluation of the various extract fractions. After most of the very rare and valuable mushroom material had been used, and the extracts tested in animals without unequivocal results, some doubt arose as to whether the mushrooms cultivated and dried in Paris were still active at all. In order to settle this fundamental point, I decided to test the material in question on myself. I ate 32 dried specimens of Psilocybe mexicana weighing 2.4 grams, corresponding to a medium dose by Indian standards. The mushrooms exerted a marked psychotomimetic effect. The following is a translation of my original report in German on this experiment, which took place on July 1, 1957:
Thirty minutes after my taking the mushrooms, the exterior world began to undergo a strange transformation. Everything assumed a Mexican character. As I was perfectly well aware that my knowledge of the Mexican origin of the mushroom would lead me to imagine only Mexican scenery, I tried deliberately to look on my environment as I knew it normally. But all voluntary efforts to look at things in their customary forms and colors proved ineffective. Whether my eyes were closed or open, I saw only Mexican motifs and colors. When the doctor supervising the experiment bent over me to check my blood pressure, he was transformed into an Aztec priest and I would not have been astonished if he had drawn an obsidian knife. In spite of the seriousness of the situation, it amused me to see how the Germanic face of my colleague had acquired a purely Indian expression. At the peak of the intoxication, about 1 Vi hours after ingestion of the mushrooms, the rush of interior pictures, mostly abstract motifs rapidly changing in shape and color, reached such an alarming degree that I feared that I would be torn into this whirlpool of form and color and would dissolve. After about six hours the dream came to an end. Subjectively, I had no idea how long this condition had lasted. I felt my return to everyday reality to be a happy return from a strange, fantastic but quite real world to an old and familiar home.
This personal study showed that the negative results of the tests in animals were due not to inactivity of the mushroom material, but to insensitivity of the animal assay, and that human beings provided a more sensitive index of substances with psychic effects than did animals. Since we had no other way to separate the active fractions from the various extracts than by gauging their psychic effects in man, we had no other choice than to test our extracts on ourselves if we wished to continue these investigations. Based on my self-experiment, in which 2.4 g of the dried mushrooms had provoked a strong experience lasting several hours, we decided to use, in the examination of our extract fractions, equivalents corresponding to only one third of this quantity, that is to 0.8 g dried material. At this level, a mild but still clear-cut psychic reaction was elicited, if the fraction contained the active principles. My co-workers and some of my colleagues participated in this test series as ‘guinea pigs. ’
With the aid of this reliable test on human beings it was possible to extract the active principles from the mushroom and to purify and, finally, crystallize them in the following way.
In order to preserve the possibly very sensitive, active principles, we used only neutral solvents and the extractions were carried out at room temperature. After the extraction of the finely-ground mushrooms with chloroform, with benzene, and with acetone, the whole activity was still in the mushroom material. The active principles were easily and completely extracted with methanol. From the residue of this extract, inactive constituents could be eliminated by treatment with chloroform. The remaining easily water-soluble preparation was purified by precipitation of a concentrated solution in water with ethanol. The activity remained in the filtrate. The residue of the evaporated filtrate contained the active principles enriched a hundred fold compared to the dried mushrooms. A further concentration of the active constituents was possible by paper chromatography. Using Whatman-I-paper with water-saturated butanol as solvent, four zones were obtained, the nature of which was determined by cutting the chromatograms into small strips, extracting the single strips with methanol, and weighing the residues. In one of the four bands, the whole activity was found in the form of an easily water-soluble, halogen-containing powder. After treatment with silvercarbonate, elimination of silver ions with H2S, and concentration of the aqueous solution in vacuo, the substance crystallized in fine white needles. With the few milligrams obtained in this way we made several tests. The new psychotropic principle, which was named ‘psilocybin,’ elicited a violet color with Van Urk-reagent, characteristic for indole derivatives.
For the subsequent isolation experiments, we could rely on this color test. When paper chromatograms prepared as described above, were sprayed, after having been dried, with a solution of p-dimethylaminobenzaldehyde in benzol and put in an atmosphere of dry HC1 gas, psilocybin produced a violet spot with Rf 0.1. A weaker spot with a blue color and Rf 0.5 was observed, corresponding to a second active principle, which we named ‘psilocin. ’
For the preparation of larger quantities of psilocybin and psilocin, sclerotia-containing mycelia of P. mexicana could be used, which my colleagues Drs. A. Brack and H. Kobel were able to produce with good yield in the laboratory on a large scale. In the preparative experiments, the final purification on paper chromatograms was replaced by column chromatography. We used columns of cellulose powder with water-saturated butanol as solvent. With this procedure it was possible to prepare several grams of psilocybin and several centigrams of psilocin, enough for the elucidation of their chemical structures. Both compounds were purified by recrystallization from methanol, from which they separated in characteristic crystals. (Figure 1).
The yield of psilocybin from dried carpophores of P. mexicana was 0.2-0.4%, from dried sclerotia-containing mycelium 0.2- 0.3%. Psilocin was found only in trace amounts.
The following findings were indicative for the determination of the chemical structure:
*The UV-spectra of psilocybin and psilocin showed the characteristics of 4-hydroxylated indole derivatives. Probably in no other laboratory in the world would there have been 4-hydroxy indole for comparison purposes. We had this compound on our shelves because of our work on the synthesis of lysergic acid, which is one of the very few natural indole compounds with a substituent at the 4 position. For the total synthesis of psilocybin and psilocin, which followed the elucidation of their structures, the starting material, that is, 4-hydroxy indole, was already at hand. The mushrooms had fallen into just the right laboratory, well prepared for the investigation of their active principles.
‘Psilocybin, with the empirical formula C12H17O4N2P, hydrolyzed in aqueous solution at 150° C. in an atmosphere of nitrogen into psilocin and 1 equivalent of phosphoric acid. Psilocybin, the first, and hitherto only known, naturally-occurring indole compound bearing a phosphoric acid radical, turned out to be the phosphoric acid ester of psilocin.
‘Treatment of psilocybin with diazomethane yielded dimethylpsilocybin, a compound with betaine character. Pyrolysis of this derivative in a high vacuum at 280-290 °C. split off trimethylamine. Psilocybin itself did not give this reaction, indicating that it contained only a dimethylamino group.
Mainly from these findings, and considering that nearly all natural indole compounds contain a tryptamine radical, it could be concluded that psilocybin would possess the structure of 4- phosphoryloxy-N,N-dimethyltryptamine and psilocin of 4- hydroxy-N,N-dimethyltryptamine. The correctness of these structures was demonstrated by the total synthesis of psilocybin and psilocin, according to the procedure illustrated in the following scheme (Figure 2).
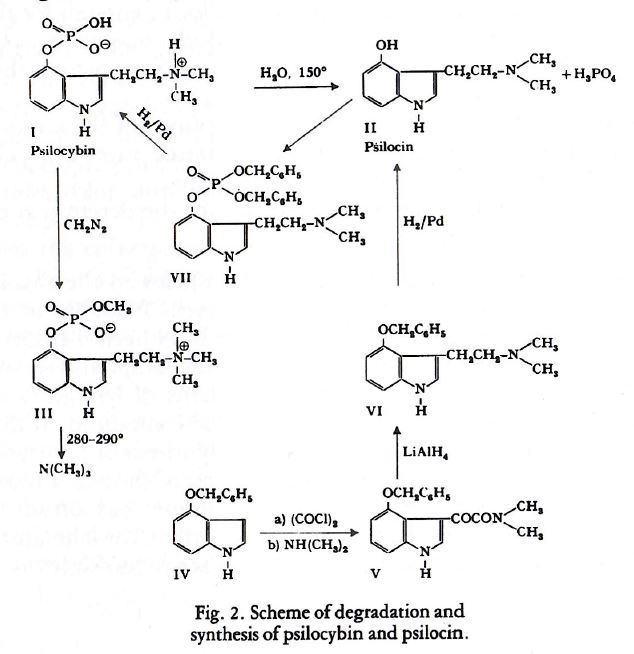
4-Benzyloxy-indole, which we had at our disposal from earlier investigations, was reacted with oxalylchloride. The resulting compound was not isolated, but treated immediately with dimethylamine to provide 4-benzyloxyindolyl-(3)-gyoxylic acid dimethylamide (V). The carbonyl grouping of V was reduced with lithiumaluminumhydride to yield 4-benzyloxy-N,N-di- methyltryptamine (VI) which after elimination of the benzyl radical gave 4-hydroxy-N,N-dimethyltryptamine (II), identical with natural psilocin. Phosphorylation of psilocin with dibenzylphosphorylchloride yielded VII, which after debenzylation with H2/Pd gave 4-phosphoryloxy N,N-dimethyltryptamine, identical with natural psilocybin.
The yields in all steps are good. The synthetic preparation of psilocybin and psilocin is therefore much cheaper and more rational than extracting these compounds from the mushrooms (6).
This was the unveiling of the riddle of the sacred mushrooms. The compounds, the magic psychic effects of which made the Indians believe for thousands of years that a god was residing in the mushrooms, could now be synthesized in the test tube.
Later other species of Psilocybe belonging to the teonandcatl group were also shown in our laboratory to contain psilocybin, usually along with a small amount of psilocin, for example, P. caerulescens Murrill var. Mazatecorum Heim, P. Zapotecorum Heim, P. Aztecorum Heim, P. semperviva Heim and Cailleux, and lastly Strop haria cubensis Earle (2).
Following our work, American scientists Tyler (9), Benedict (1), and others have analyzed other Psilocybe species that are not used for magical purposes, and species of other botanically closely-related genera, and have found these to contain psilocybin, psilocin, and derivatives thereof—but these important investigations are beyond the scope of this chapter.
The availability of the active principles of the hallucinogenic mushrooms in the form of pure chemical compounds made it possible to study their pharmacological properties and mental effects. For access to the basic pharmacological and medical literature, reference must be made to review articles (4,5).
Psilocybin and psilocin produced psychotomimetic effects in man that are similar to those produced by mescalin or LSD. The medium oral dose for man is 6 to 8 mg; corresponding to the consumption of about 2 g of dried Psilocybe mexicana. The
TEONANACATL: HALLUCINOGENIC MUSHROOMS OF NORTH AMERICA mushroom principles are about 100 times more active than mescalin and possess about 1/100 of the activity of LSD. Psilocybin and psilocin in equimolar doses have the same strength, from which it can be deduced that the phosphoric acid radical of psilocybin is of no importance to the hallucinogenic activity. Psilocybin is a stable compound which is readily soluble in water. Psilocin, on the other hand, being a compound with a free phenolic group, is very sensitive to oxidation and difficultly soluble in water. It seems, therefore, that the phosphoric acid radical of psilocybin has mainly a protective function.
When a novel pharmacologically active principle is discovered, chemists start to modify its structure in order to find out the structural prerequisites for the activity and in the hope of finding derivatives with improved pharmacological action. Such a research project has been carried out in our laboratory with psilocybin and psilocin. A large number of derivatives and modifications have been prepared and tested pharmacologically, and some have also been submitted to clinical evaluation (8). The main insight into the relationship between molecular structure and psychotropic action of this group of substances that resulted from these investigations was that the highest psychotropic activity is found in the tryptamine derivatives which bear the hydroxyl or phosphoryloxy function at the 4 position. Isomers of psilocybin and psilocin with the hydroxyl function at the 5,6, or 7 position were much less active or inactive. Another interesting result was the observation that the diethyl analogues of psilocin and psilocybin, that is, 4-hydroxy-N,N-diethyltrypta- mine and 4-phosphoryloxy-N,N-diethyltryptamine (called CZ- 74 and CY-19 respectively), possessing qualitatively the same activity, differ from psilocin and psilocybin in that their duration of action is somewhat shorter, amounting to an average of 3 ‘A hours, compared with 4 to 6 hours for psilocybin and psilocin (7).
My practical chemical investigations on the active principles of the sacred mushrooms of Mexico came to an end after the article on the modifications of psilocybin and psilocin was published in 1959. This work was carried out with my colleagues F. Troxler and F. Seeman (8). Later, in 1962, my mushroom studies found a second, adventurous and convincing conclusion when I made an expedition with my friend Gordon Wasson to Mexico.

I met R. Gordon Wasson personally for the first time in 1959, after having been in correspondence with him since the beginning of our collaboration on the investigation of the magic mushrooms. After we had succeeded in isolating, characterizing, and synthesizing the active principles, Gordon Wasson visited the Sandoz laboratories where this work had been accomplished. He was delighted to see the very essence of his mushrooms in the form of white crystals (Figure 3). On this occasion a joint lecture was presented to the Sandoz management and invited guests: Gordon Wasson on the ethnomycological aspects; Dr. Aurelio Cerletti, my colleague of the medical department, on pharmacology; and myself on the chemistry of the magic mushrooms.
My friendship and collaboration with Gordon Wasson resulted in the investigation of two other magic drugs of Mexico. We
were able to resolve the riddle of ololiuhqui, where lysergic acid amides closely related to LSD and other ergot alkaloids were found to be the active principles. There was still the problem of another Mexican ceremonial drug, called pipiltzintzintli in the old codices and, in Spanish, ‘hojas de la Pastora’ (leaves of the shepherdess). The plant had not yet been chemically analyzed, nor yet even identified botanically. In order to collect authentic plant material for botanical identification and chemical analysis, Gordon Wasson organized an expedition in September and October 1962 into the mountains of southern Mexico where the ‘hojas’ were known to be used by shamans, and invited me and my wife and Mrs. Irmgard Weitlaner-Johnson, the widow of the late American ethnologist J. B. Johnson, to participate.
We had an adventurous trip on muleback through the Sierra Mazateca with an Indian guide, two Indian boys who cared for the mules, and a young Mazatec woman who served as interpreter. On the way, we participated in a nocturnal ceremony with a curandera who used and distributed the ‘hojas,’ gathered our plant material for botanical identification, and arrived at Huautla de Jimenez, Oaxaca. Here we visited the famous curandera Maria Sabina, who in that historical ceremony in 1955 had initiated Wasson into the sacred mushroom cult. Gordon asked her for a consultation and explained to her, through our interpreter, that we had brought the ‘spirit’ of the mushrooms in the form of pills which she could use, because at that time there were no mushrooms available.
On the evening of Thursday, October 11, 1962, we gathered on the veranda of the hut of Dona Herlinda, our Mazatec interpreter. Maria Sabina was already there with her two daughters, Polonia and Aurora, and other people of her clan. Don Aurelio Garcia, a famous 79 year old curandero, blind in one eye, very tall, and very strong, was also present and eager to try the magic pills. Chocolate and sweets were served. Later everyone withdrew into the hut. Everybody tried to get comfortable on mats on the floor or on some kind of bed.
After about an hour, when many were already sleeping, Maria Sabina started the ceremony, lit a candle on an improvised altar, and began to pray and burn copal (p resinous incense). At about 11 o’clock the pills (each containing 5 mg synthetic psilocybin) were distributed, after Maria Sabina had held them ceremonially over the copal vessel. I had suggested that Maria Sabina take
two pairs of pills (since the mushrooms are always administered in pairs). The same dosage, that is, 20 mg of psilocybin, was given to her daughter Polonia, who also served as a curandera, and to Don Aurelio. Aurora got one pair. Gordon also took one pair, my wife and Irmgard only one pill each. I myself tried the juice of the ‘hojas de la Pastora,’ prepared for me ceremonially by a young girl.
After everybody had taken the drug, the candlelight was extinguished and everyone awaited the effects in the darkness. About 20 minutes after the ingestion there was some murmuring between Maria Sabina and Don Aurelio. Our interpreter told us that they said the pills did not work. There came to be considerable agitation in the room. Gordon (who was lying near me) and I were discussing the situation. For us it was clear that the onset of the effects of the pills, which must dissolve in the stomach before they can be absorbed, takes place only after 30 to 45 minutes, in contrast to the mushrooms which, when chewed, work faster because part of the drug is absorbed immediately by the mucosa in the mouth. But how could we have given a scientific explanation under such conditions? Instead of trying to explain, we decided to act. In order to quiet the situation some more pills were distributed. The curanderas and the curandero each got an additional pair. They now had taken a total dosage of 30 mg of psilocybin. After about ten more minutes the mushroom spirit began to work. Maria Sabina started to chant, to pray, lit the candle, handled the copal. Her daughters and Don Aurelio joined in chanting and praying. After midnight had passed Maria Sabina began soothsaying, answering the questions Gordon and my wife had asked her. She said that Gordon’s daughter, who had had to enter the hospital to give birth just when Gordon had left New York for the expedition to Mexico, was well and so was the baby. My wife, who had been afraid to leave her very old parents, was comforted by the curandera, who told her that they were well and would live many more years. Both predictions turned out to be true.
The ceremonial handling of the candle, the incense vessel, of certain herbs, the chanting, hand clapping, and praying lasted the whole night. Some of the participants were sleeping when the light of the new day signalled an end to the session.
At dawn, when we left the hut, Herlinda, our Mazatec interpreter, told us that Maria Sabina had said that there was no
TEONANACATL: HALLUCINOGENIC MUSHROOMS OF NORTH AMERICA difference between the pills and the mushrooms. This was the final proof that our synthetic psilocybin was identical in every respect to the natural product.
In order to express our gratitude to Maria Sabina for this gala performance, I gave her a bottle of the pills, labelled ‘Indo- cybin,’ which is the generic name for psilocybin. The prefix ‘Indo-’ refers to the Indians, the original discoverers of this drug, or to the chemical indole, of which psilocybin is a derivative. Maria Sabina expressed her thanks for the gift, saying that she would now be able to serve people even when no mushrooms were available.
Albert Hofmann
Burg i.L. , Switzerland 12 December 1977
BIBLIOGRAPHY
- Benedict, R.G., L.R. Brady, A. H. Smith and V. E. Tyler. Occur- ence (sic) of psilocybin and psilocin in certain Conocybe and Psilo- cybe species. Lloydia ly. 156-159, 1962.
- Heim, R. and R. G. Wasson. Les Champignons Hallucinogenes du Mexique. du Museum National d’Histoire Naturelie, Paris, 1958.
- Hofmann, Die Mutterkomalkalmde. Ferdinand Enke Verlag, Stuttgart, 1964.
- Hofmann, A. Psychotomimetic Agents. In A. Burger: Chemical Constitution and Pharmacodynamic Action. II: Drugs Affecting the Central Nervous System. M. Dekker, New York, 1968.
- Hofmann, A. Teonanacatl and Ololiuqui, two ancient magic drugs of Mexico. Bulletin on Narcotics 23: 3-14, 1971.
- Hofmann, A., R. Heim, A. Brack, H. Kobel, A. Frey, H. Ott. Th. Petrzilka and F. Troxler. Psilocybin und Psilocin, zwei psychotrope Wirkstoffe aus mexikanischen Rauschpilzen. Helvetica Chimica Acta to-. 1557-1572, 1959-
- Leuner, H. and G. Baer. Proceedings IV. Meeting Collegium Internationale Neuropsychopharmacologicum, Birmingham, 1964.
- Troxler, F., F. Seeman and A. Hofmann. Abwandlungsprodukte von Psilocybin und Psilocin. Helvetica Chimica Acta 42: 2073- 2103, 1959.
- Tyler, V.E. Indole derivatives in certain North American mushrooms. Lloydia 24: 71-74, 1961.
- Wasson, V. P. and R. G. Wasson. Mushrooms, Russia and History. Pantheon, New York, 1957.