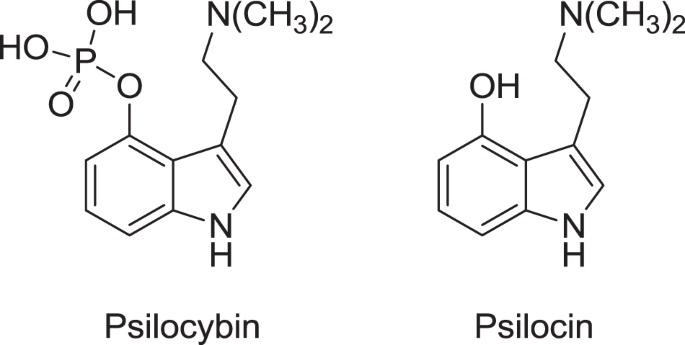
Psilocybin and psilocin are closely related to baeocystin (= 0-phosphoryl-4-hydroxy-N-methyltryptamine, norpsilocybin), which probably represents the biogenic precursor of psilocybin (Repke et al. 1977; cf. also Brack et al. 1961 and Chilton et al. 1979). Baeocystin may be a derivative of tryptophan (Brack et al. 1961).
Psilocybin (4-phosphoryloxy-N,N-dimethyltryptamine) is an indole-based secondary metabolite produced by numerous species of mushrooms.
South American Aztec Indians referred to them as teonanacatl, meaning “god’s flesh,” and they were used in religious and healing rituals. Spanish missionaries in the 1500s attempted to destroy all records and evidence of the use of these mushrooms. Nevertheless, a 16th century Spanish Franciscan friar and historian mentioned teonanacatl in his extensive writings, intriguing 20th century ethnopharmacologists and leading to a decades-long search for the identity of teonanacatl.
Their search ultimately led to a 1957 photo-essay in a popular magazine, describing for the Western world the use of these mushrooms. Specimens were ultimately obtained, and their active principle identified and chemically synthesized.
In the past 10–15 years several FDA-approved clinical studies have indicated potential medical value for psilocybin-assisted psychotherapy in treating depression, anxiety, and certain addictions. At present, assuming that the early clinical studies can be validated by larger studies, psilocybin is poised to make a significant impact on treatments available to psychiatric medicine.
Psilocybin: CY-39, indocybin, 0-phosphoryl-4hydroxy-N,N dimethyltryptamine, 3(2-dimethylamino) ethylindol-4-01 dihydrogenphosphatester
Psilocin: 4-hydroxy-N,N-dimethyltryptamine, psilocine, psilocyn (misspelling in the legal literature), 3- [2-(dimethylamino)ethyl]-1H-indole-4-01
Empirical formula: C12H17N204P (psilocybin), C12H16N20 (psilocin)
Substance type: tryptamines, indole amines (indole alkaloids)
Psilocybin was first isolated from Psilocybe Mexicana and identified by Albert Hofmann in 1955 (Hofmann et al. 1958, 1959). The phosphorylated indole amine psilocybin is transformed into psilocin by splitting off the phosphoric acid group (Hofmann and Troxler 1959). Because the protection the phosphoric acid would provide is lacking, psilocin easily oxidizes with the phenolic hydroxyl group, resulting in blue quinonoid products.
